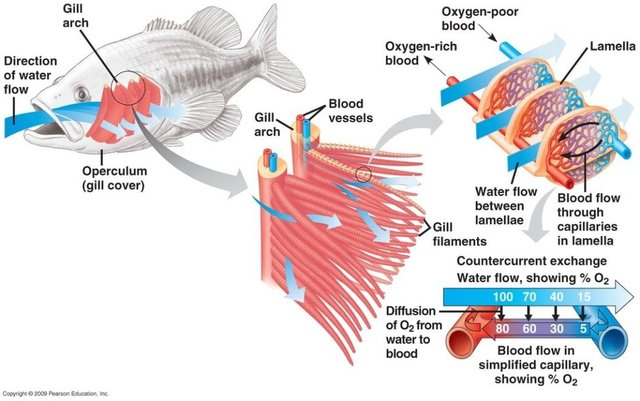
When you picture a freshwater ecosystem it really goes one of two ways, largely there is the group that look for a heavily planted water body with a diverse number of species or something that has a lot of leaf litter almost entirely complete for several layers.

What if I told you this isn’t entirely true, a half truth or some what out of date?
There is a lot to unpack here.
- The reality of freshwater function
- The reality of freshwater habitat diversity
- The deception of anthropomorphic effects
- Source of nutrients
- Why does algae do so well but plants don’t?
- What is algae?
- Long live algae! Leaf litter is overrated.
- Leaf litter is not all bad
- Is there hope for algal growth?
- Aquariums are anthropogenic
The reality of freshwater function
Freshwater habitats are very diverse, this is largely based on the locality of the habitat within the river.
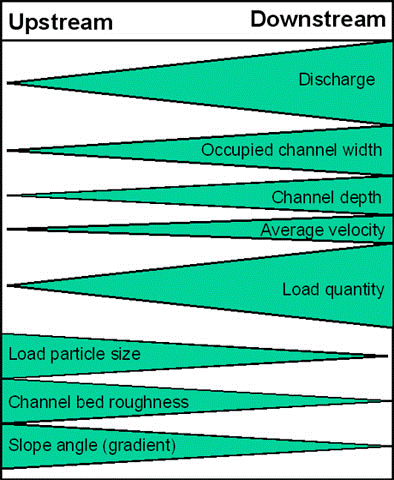
The Bradshaw model (Fig 1) explains this well but being largely based on temperate, North American and European ecosystems it doesn’t reflect tropical systems particularly as well.
The struggle for plant life.
When thinking about plants load particle size is important, plants in freshwater ecosystems largely need something to root onto and this isn’t found upstream where sediment is quickly pushed downstream. Downstream though this sediment load, load particle size is the opposite but because it’s remaining in the water column it becomes what is known as having a high turbidity (low visibility), this means less light can pass through the water and reduces photosynthesis. This is why so many aquarium plants are grown ‘terrestrially’ or aquaponically because they aren’t naturally aquatic. Aquatic plants have a struggle and there are solutions such as developing very strong roots and a reduced amount of structural support as if there is less sediment and a higher flow those stems are highly likely to break. In high turbidity waters that’s where we see the water lilies, Nymphaea spp., Nymphoides but also more floating plants who reach for the surface where they can receive light.

Some plants might have to deal with extreme drought in a dry season and then being drowned in the wet season. This is where we seem to see some really extremophile plants such as some Podostemaceae in the Rio Xingu likely of the genus Morera as reported by Peter Peterson in Amazonas (Fig 2).
Where is the leaf litter?
This is such a similar story for leaf litter as deposition, and particle size largely depends on flow rate but position in the river. Firstly you require that river to be surrounded by trees but also that the current is weak enough that the leaves can just fall to the bottom. When you look at rivers like the Rio Xingu, Rio Orinoco or towards the Rift Valley this is not shown in the major areas of the rivers or lakes where many of our fishes are caught. Hillstreams where other fishes are caught if leaves and fruits fall then the current might be so strong it’ll push it downstream to be deposited in certain areas of a meander known as deposition zones.

This makes it just really complex to generalize but this is also why some areas have high tannin composition, Rio Negro and others have none such as the main channel of the Amazon and where they meet is known as the meeting of waters. The Rio Negro’s distinct black waters are likely due to it’s geology (Sioli, 1968), but likely those contributing tributaries cause a lot of the difference. Are these tributaries hillstream’s and likely to be less surrounded by plants at those higher elevations? Or maybe that geology largely contributes to the types of trees surrounding smaller source streams? There is a lot to unpack about why, but as hobbyists I’m not sure this why really matters? What matters is what these water bodies are and why what they are effects our fishes.
The reality of freshwater habitat diversity
It is a little bit of a pain but the image we have of freshwater habitats is curated by the idea perpetuated by brands and hobbyists. There is the idea that black water, therefore leaf litter is so common and when we look at the rivers and lakes around us, surely we must notice the difference?
Rivers are generally classified into white water or black water and then subdivided further as explained so clearly by Bogotá-Gregory et al. (2020; fig. 3).
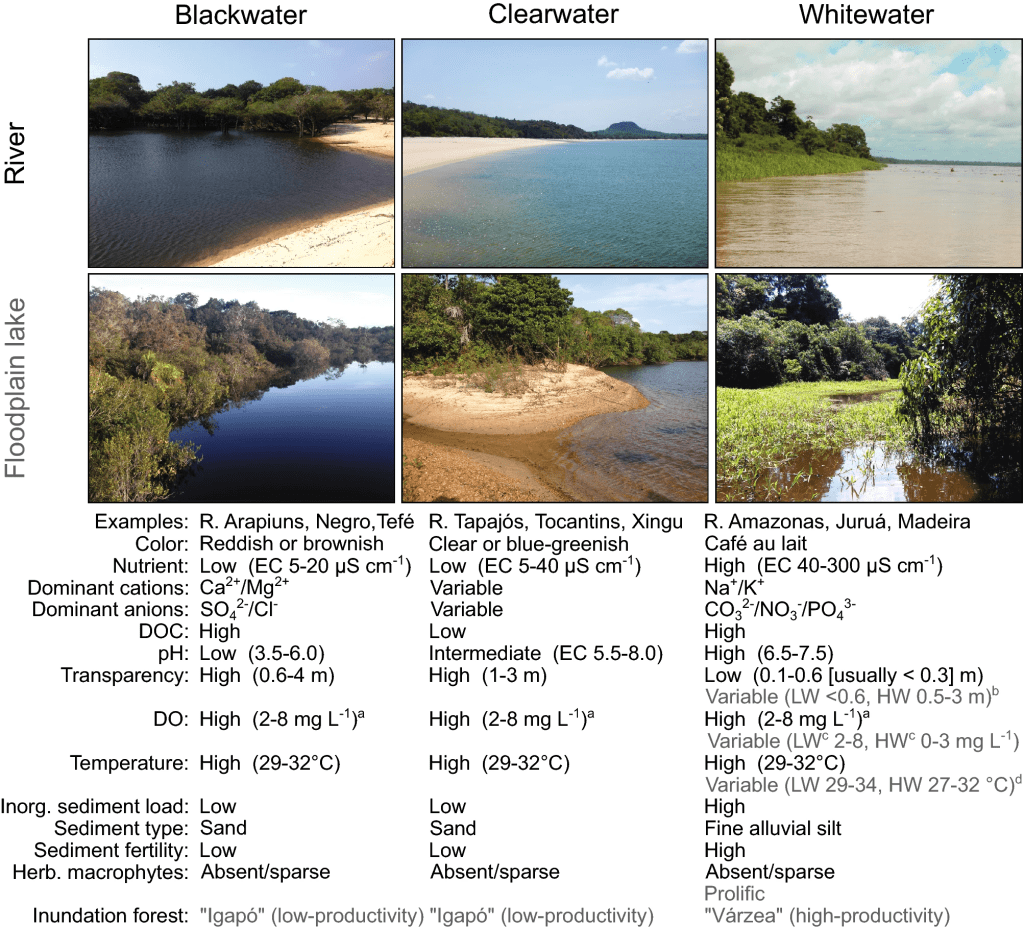
Do I need to say more? I wonder if the influence of these ideas of freshwater ecosystems comes from the passionate fishkeepers focused on those smaller niche fish such as wild Betta (not domestic Betta splendens), other anabantoids and what I some what try to affectionately say swimmy fishes. Previously these black water, leaf litter tanks would have been seen as dirty but they have effectively justified this ecosystem for the hobby. The problem has come where it’s shoved almost all freshwater species into being black water, particularly if from South America.
Lakes also show a wide amount of diversity between lakes and within that same lake there can be many different habitats.
The deception of anthropomorphic effects
As humans encroach on land and change it, the function changes too. This has been happening for thousands of years but regardless this effects the species that inhabit these waters. Rivers have been straightened and moved, stopped and redirected or even slowed and sped up. New lakes are created and others destroyed. Our waste thrown into rivers and lakes whether it be effluent or invasive species, warm water from coolant systems or mining waste. Freshwater is changing and fishes cannot always adapt fast enough. Your local river might not always be representative of nature, the plants are likely invasive and the sediment is likely due effluent or waste. The presence of a dam or weir means that the entire flow of the river has changed.

This can be similar as to when we look at wild habitats, but much of the footage and images we see today seem to have been taken a while ago. Still the footage and photos of the Rio Xingu is showing that amount of sediment that might be connected to the building of the Belo Monte dam in 2011.
Source of nutrients
This is not where the debate is in the hobby but in the science it certainly is (Neres‐Lima et al., 2017; Hamilton et al., 1992). The long held belief in the hobby is that nutrients originates from leaf litter, fruit, branches etc. Therefore it’s important in the aquarium as some how all fishes even if there is no leaf litter in their habitat benefit from it.
This idea that allochthonous (leaf litter, fruit, branches, other botanicals etc.) nutrients is the major source of nutrients over autochthonous (algaes) can be true in some ecosystems but it doesn’t mean that nutrients is accessible (Thorp & Delong, 2002) and therefore few organisms obtain nutrients from it. This theory of nutrients deriving from allochthonous relies on the presence of leaf litter, botanicals being present in the water or further upstream and in shaded rivers it might contribute where no algae’s are found (Neres-lime et al., 2017). One answer answer is black water, often characterized by high botanicals/allochthonous influences is much lower in productivity and if a source of nutrients this should be another story (Bogotá-Gregory et al., 2020; Lewis et al., 1988). We also know as hobbyists algae’s can grow in very dimly lit aquariums though, nutrients is a whole other discussion.
It doesn’t seem an easy question to answer as while isotopes seem to be an answer and algae’s contribute most to nutrients in one study (Hamilton et al., 1992). As the cited papers suggest rivers flowing through slower flowing areas might contribute to nutrients opposed to it being directly in the rivers, but this means that the nutrients is in the source water not where the fishes are.
Why does algae do so well but plants don’t?
Algae’s are found in all of these habitats but to varying amounts, many do require light as photosynthetic organisms but some are known as heterotrophs so can gain energy by other means. It’s not just algae’s but other microbes that grow in these ecosystems, all of these unlike plants cling to surfaces and in a very small size meaning that there is much less drag. If they do grow too big then parts get broken off to throw downstream. What might be forgotten is bryophytes also do this but still photosynthetic. This leaves sponges as filter feeders.
What is algae?
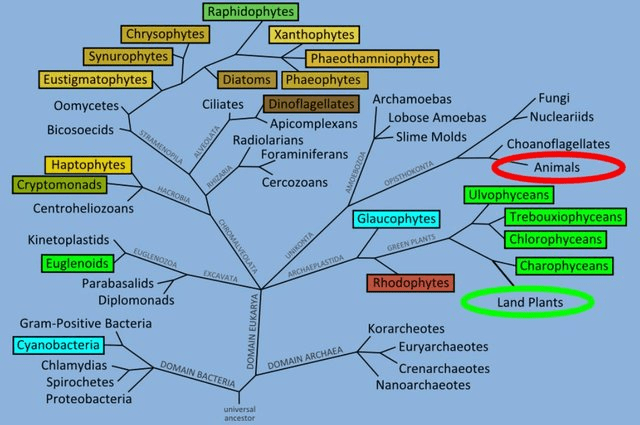
Algae (Alga, singular) is basically a common name for a whole mixture of different organisms (Milledge, 2013). Some hobbyists will exclude Cyanobacteria as it is a bacteria yet it makes little sense given this clade is already polyphyletic (Fig 5), a real pick and mix. Another opinion is as these organisms are photosynthetic they must be similar to plants, plants particularly vegetables make good nutritional replacements. As you can see from the above it’s unlikely plants make up for algae’s nutritionally (Čengić-Džomba et al., 2022; Nagappan et al., 2021; Vucko et al., 2017).
This makes algae such a diverse grouping to describe as earlier stated some being heterotrophs do not just obtain their ‘energy’ from the sun and others might obtain it from feeding or even parasitism e.g. dinoflagellates (Levy et al., 2007; Sudhagar et al., 2022).
Algae is so important to an ecosystem and I will include other microbial life on that. This wide diversity of organisms feature in many fishes diets even making the majority of many Loricariid diets (Lujan et al., 2015). There is a link between algae’s and the fishes that feed on them influences the habitat’s and those fishes (Power, 1984).
Algae’s have to compete with different microbes and this is fascinating in terms of succession. Succession is the process of a habitat maturing over time with the change in species present. This happens in an aquarium over time but also how we maintain our aquariums seems to effect this. Generally you can expect as a grazing fish moves over an area it removes all the organisms it feeds best on, leaving an opening for other species of microbes to grow. These original fishes might not feed on that new microbes but other fishes will have and could this be where niche partitioning further occurs.

Long live algae! Leaf litter is overrated.
You don’t need a green aquarium to embrace them, realistically if you’re getting high amounts of algae it could infer high levels of a compound or element that encourages their growth as I keep stating.
Above where I mention hypothetical niche partitioning regarding algae’s and other surface growing microbes. This will inevitably occur with bacterial films or any growths on leaf litter, if you’re seeing the films on botanicals in general for a while they probably aren’t being eaten by anything and when they eventually go it could be due to exhausting the source of nutrients. I particularly noted this when experimenting with different woods and palms, if these biofilms were being eaten there wouldn’t be so much biofilm’s growing. So far in Loricariids at least we know this niche partitioning is largely in where food items are located (Lujan et al., 2011) and while their diets aren’t the best studied we do have a realistic idea that they are not all feeding on the same algae’s or microbes (Lujan et al., 2012; Valencia & Zamudio, 2007; Delariva & Agostinho, 2001).
It is frequently stated that leaf litter has beneficial properties due to tannins but it is rarely mentioned that tannins are antinutrients and can cause physiological damage (Omnes et al., 2017; Li et al., 2020; Maulidiyah et al., 2019). Given some fishes inhabit these naturally they likely do have physiological mechanisms to manage tannins within the water, although given many fishes do have negative physiological responses as cited above not all do.
The frequent dislike of tea tree oils in the hobby is contrasted with the support for tannins having antimicrobial properties and they possibly do but in prevention and handling of disease in fishes, these effects are minimal (Imperatore et al., 2022). Perhaps the bias against tea tree oils is due to a dislike of brands as a review by Valladão et al. (2015) infers that use of known concentrations of compounds of plant origins (herbal treatments) can show very effective use. Leaf litter and tannins from the leaf litter have a flaw here, species identification and in using the plant as a whole it is difficult to say what compounds and concentrations are being added. We are living in an anti-pharmaceutical age and who can tell us more about how compounds interact with physiology then pharmacologists as the story is quite complex.
Leaf litter is not all bad
Leaf litter and botanicals can add great hiding spaces for smaller fishes, there is still a number of fishes who find it as a part of their natural habitat and this is worth replicating. The main downfall with leaf litter is siphoning to remove waste and therefore excess nutrients, other heavier botanicals would be more ideal if this is a big concern. But without evidence we shouldn’t jump to conclusions.

Is there hope for algal growth?
No doubt algae does actually struggle to grow to much of an extent in a mature aquarium that isn’t constantly being scrubbed. For an aquarium which might benefit from ‘naturally’ growing algae’s such as for feeding fishes there is hope. We can’t use sunlight like outside but perhaps using UVB lighting could encourage growth. Most of our aquarium algae growth relies on nutrients but outside sunlight would perhaps contribute, something I’d certainly like to explore more.
Aquariums are anthropogenic
At the end of the day aquariums are anthropogenic and the argument about the importance of leaf litter vs algae really stall’s there. Algae’s are generally great indicators of nutrients, generally different algae’s hint to the age of an aquarium.
At the end maybe the promotion of leaf litter is easy to justify as natural then algae. There are few true biotopes and few ever show algae’s but the scale you’d need to grow them would be insane.
References:
Delariva, R. L., & Agostinho, A. A. (2001). Relationship between morphology and diets of six neotropical loricariids. Journal of Fish biology, 58(3), 832-847.
Bogotá-Gregory, J. D., Lima, F. C., Correa, S. B., Silva-Oliveira, C., Jenkins, D. G., Ribeiro, F. R., … & Crampton, W. G. (2020). Biogeochemical water type influences community composition, species richness, and biomass in megadiverse Amazonian fish assemblages. Scientific Reports, 10(1), 15349.
Čengić-Džomba, S., Džomba, E., & Hadžić, D. (2022). An Overview of Using Algae Meal in Feeding Freshwater Fish Species. In Scientific-Expert Conference of Agriculture and Food Industry (pp. 171-182). Cham: Springer Nature Switzerland.
Chaves, M. S., Oliveira, R. R., Gonçalves, A. P., Sousa, L. M., & Py-Daniel, L. H. R. (2023). A new species of armored catfish of the genus Scobinancistrus (Loricariidae: Hypostominae) from the Xingu River basin, Brazil. Neotropical Ichthyology, 21(3), e230038.
Farha, A. K., Yang, Q. Q., Kim, G., Li, H. B., Zhu, F., Liu, H. Y., … & Corke, H. (2020). Tannins as an alternative to antibiotics. Food Bioscience, 38, 100751.
Hamilton, S. K., Lewis, W. M., & Sippel, S. J. (1992). Energy sources for aquatic animals in the Orinoco River floodplain: evidence from stable isotopes. Oecologia, 89, 324-330.
Imperatore, R., Fronte, B., Scicchitano, D., Orso, G., Marchese, M., Mero, S., … & Paolucci, M. (2022). Dietary supplementation with a blend of hydrolyzable and condensed Tannins ameliorates diet-induced intestinal Inflammation in Zebrafish (Danio rerio). Animals, 13(1), 167.
Küchler, I. L., Miekeley, N., & Forsberg, B. R. (2000). A contribution to the chemical characterization of rivers in the Rio Negro Basin, Brazil. Journal of the Brazilian Chemical Society, 11, 286-292.
Levy, M. G., Litaker, R. W., Goldstein, R. J., Dykstra, M. J., Vandersea, M. W., & Noga, E. J. (2007). Piscinoodinium, a fish-ectoparasitic dinoflagellate, is a member of the class Dinophyceae, subclass Gymnodiniphycidae: convergent evolution with Amyloodinium. Journal of Parasitology, 93(5), 1006-1015.
Lewis Jr, W. M. (1988). Primary production in the Orinoco River. Ecology, 69(3), 679-692.
Li, M., Feng, L., Jiang, W. D., Wu, P., Liu, Y., Jiang, J., … & Zhou, X. Q. (2020). Condensed tannins decreased the growth performance and impaired intestinal immune function in on-growing grass carp (Ctenopharyngodon idella). British Journal of Nutrition, 123(7), 737-755.
Lujan, N. K., German, D. P., & Winemiller, K. O. (2011). Do wood‐grazing fishes partition their niche?: morphological and isotopic evidence for trophic segregation in Neotropical Loricariidae. Functional Ecology, 25(6), 1327-1338.
Lujan, N. K., Winemiller, K. O., & Armbruster, J. W. (2012). Trophic diversity in the evolution and community assembly of loricariid catfishes. BMC Evolutionary Biology, 12, 1-13.
Maulidiyah, V., Sulmartiwi, L., & Masithah, E. D. (2019). The effect of immersion time in tannin solution towards the adhesiveness and hatching degree of the eggs of common carp (Cyprinus carpio). AACL Bioflux.
Milledge, J. J. (2013). Energy balance and techno-economic assessment of algal biofuel production systems (Doctoral dissertation, University of Southampton).
Nagappan, S., Das, P., AbdulQuadir, M., Thaher, M., Khan, S., Mahata, C., … & Kumar, G. (2021). Potential of microalgae as a sustainable feed ingredient for aquaculture. Journal of Biotechnology, 341, 1-20.
Neres‐Lima, V., Machado‐Silva, F., Baptista, D. F., Oliveira, R. B., Andrade, P. M., Oliveira, A. F., … & Moulton, T. P. (2017). Allochthonous and autochthonous carbon flows in food webs of tropical forest streams. Freshwater Biology, 62(6), 1012-1023.
Omnes, M. H., Le Goasduff, J., Le Delliou, H., Le Bayon, N., Quazuguel, P., & Robin, J. H. (2017). Effects of dietary tannin on growth, feed utilization and digestibility, and carcass composition in juvenile European seabass (Dicentrarchus labrax L.). Aquaculture Reports, 6, 21-27.
Power, M. E. (1984). Habitat quality and the distribution of algae-grazing catfish in a Panamanian stream. The Journal of Animal Ecology, 357-374.
Sioli, H. (1968). Hydrochemlstry and Geology in the Brazilian Amazon Region. Volume 1, fascículo 3, 1968.
Sudhagar, A., Sundar Raj, N., Mohandas, S. P., Serin, S., Sibi, K. K., Sanil, N. K., & Raja Swaminathan, T. (2022). Outbreak of Parasitic Dinoflagellate Piscinoodinium sp. Infection in an Endangered Fish from India: Arulius Barb (Dawkinsia arulius). Pathogens, 11(11), 1350.
Thorp, J. H., & Delong, M. D. (2002). Dominance of autochthonous autotrophic carbon in food webs of heterotrophic rivers. Oikos, 96(3), 543-550.
Valencia, C. R., & Zamudio, H. (2007). Dieta y reproducción de Lasiancistrus caucanus (Pisces: Loricariidae) en la cuenca del río La Vieja, Alto Cauca, Colombia. Revista del Museo Argentino de Ciencias Naturales nueva serie, 9(2), 95-101.
Valladão, G. M. R., Gallani, S. U., & Pilarski, F. (2015). Phytotherapy as an alternative for treating fish disease. Journal of veterinary pharmacology and therapeutics, 38(5), 417-428.
Vucko, M. J., Cole, A. J., Moorhead, J. A., Pit, J., & de Nys, R. (2017). The freshwater macroalga Oedogonium intermedium can meet the nutritional requirements of the herbivorous fish Ancistrus cirrhosus. Algal research, 27, 21-31.